苯并三氧化呋咱
外观
| 苯并三氧化呋咱 | |
|---|---|
| |
| 别名 | Benzenetrifuroxan |
| 识别 | |
| CAS号 | 3470-17-5 |
| PubChem | 18982 |
| ChemSpider | 17920 |
| SMILES |
|
| 性质 | |
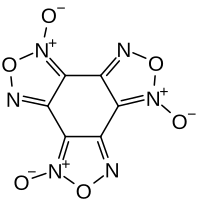
| 化学式 | C6N6O6 |
| 摩尔质量 | 252.1 g·mol−1 |
| 结构 | |
| 空间群 | Pna21[1][2] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
苯并三氧化呋咱是一种杂环有机化合物,化学式为C6N6O6。它最初于1924年由O. Turek合成,但他当时将该化合物当作六亚硝基苯。[3][4]除了亚硝基的结构外,它还可以形成对称的稠环结构。[5][1]
![1. Hexa(1-aza-2-oxaethenyl)benzene, 2. 5,7,9,11,13,15-hexaza-6,8,10,12,14,16-hexoxa-[5.5.6.2(1.4).0(3.7).0(2.9).0(13.17).0.(15.18)]-hexacycloctadec-1,3,17-triene, 3. 2,5,8,11,14,17-hexaza-3,4,9,10,15,16-hexoxa-[12.4.0.0(1.6).0(7.12)]-tricyclooctadex-6,12,18-triene](http://upload.wikimedia.org/wikipedia/commons/thumb/8/87/Benzotrifuroxan_non-real_structures.svg/401px-Benzotrifuroxan_non-real_structures.svg.png)
它可由1,3,5-三叠氮-2,4,6-三硝基苯的热分解得到。[3][4]

参考文献
[编辑]- ^ 1.0 1.1 Cady, H. H.; Larson, A. C.; Cromer, D. T. The crystal structure of benzotrifuroxan (hexanitrosobenzene). Acta Crystallographica. 1 March 1966, 20 (3): 336–341. Bibcode:1966AcCry..20..336C. doi:10.1107/S0365110X6600080X.
- ^ Maslen, E. N. A phase refinement of the crystal structure of benzotrifuroxan. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 1 September 1968, 24 (9): 1170–1172. Bibcode:1968AcCrB..24.1170M. doi:10.1107/S0567740868003912.
- ^ 3.0 3.1 O. Turek: Le 2,4,6-trinitro-1,3,5-triazido-benzene, nouvel explosif d’amorcage. In: Chimie et industrie. Band 26, 1931, S. 781–794.
- ^ 4.0 4.1 O. Turek: 1,3,5-Triazido-2,4,6-trinitrobenzen, nova inicialna vybusina. In: Chemicky obzor. Nr. 7, 1932, S. 76–79; 97–104.
- ^ Bacon, Neville; Boulton, A. J.; Katritzky, A. R. Structure of "hexanitrosobenzene" from vibrational spectroscopy. Trans. Faraday Soc. 1967, 63: 833–835. doi:10.1039/TF9676300833.
