粉防己鹼
外觀
 | |
| 臨床資料 | |
|---|---|
| 商品名 | 金艾康、漢力達等 |
| 其他名稱 | 漢防己甲素 |
| 法律規範狀態 | |
| 法律規範 | |
| 藥物動力學數據 | |
| 藥物代謝 | 肝臟 |
| 生物半衰期 | 2–7.5小時(靜脈注射)、0–26小時(口服)[2] |
| 識別資訊 | |
| |
| CAS號 | 518-34-3 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.208.615 |
| 化學資訊 | |
| 化學式 | C38H42N2O6 |
| 摩爾質量 | 622.74988 g/mol |
| 3D模型(JSmol) | |
| |
粉防己鹼[3](tetrandrine,fanchinine,hanfangchin A)是一種雙苄基異喹啉類生物鹼,可從防己科植物粉防己根中和其他中草藥提取出來[4]。具有抗炎、抗過敏、抗氧化、抗纖維化及免疫調節作用[5];其可抑制肥大細胞的脫粒,也有「類奎尼丁」的抗心律失常作用。另具有血管擴張特性,因此可以降低血壓[6]。
粉防己鹼可能具有治療肝病[7]和肝癌的潛在用途[8][9][10]。具有預防小梁切除術後結膜炎或嚴重結膜炎症患者過度瘢痕/纖維化的潛在治療價值[11]。粉防己鹼具有抗炎和抗纖維化作用,使得粉防己鹼和相關化合物可用於治療肺矽肺病,肝硬化和類風濕性關節炎。[6]還表明粉防己鹼在體外抑制埃博拉病毒進入宿主細胞,並且在對小鼠的初步研究中顯示出對抗埃博拉病毒的治療功效。[12]
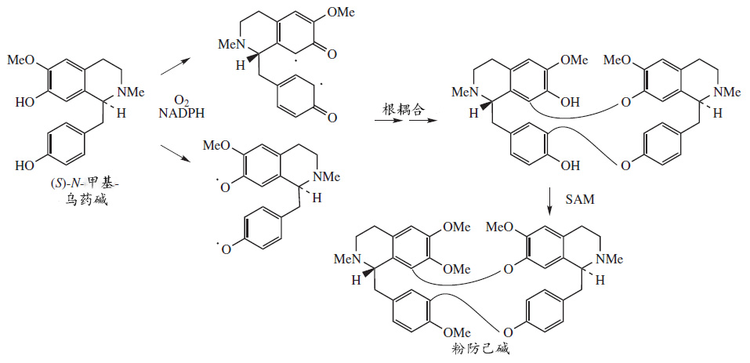
生物合成
[編輯]粉防己鹼是由S-N-甲基烏藥鹼的自由基偶聯二聚化生物合成的:[13]
同義詞
[編輯]其同義詞包括防己鹼、漢防己鹼A、NSC 77037、(S ,S )-(+)-粉防己鹼、青藤鹼A、TTD、四聯蛋白和d-粉防己鹼[14] 。用作藥品時稱為漢防己甲素[1]。
參考文獻
[編輯]- ^ 1.0 1.1 汉防己甲素片(北海阳光). 丁香園·用藥助手.
- ^ Xin-Hui Jiang, Jun-Qing Yang, Na Li, Han Wang, Qi-Xin Zhou. The pharmacokinetical study of plant alkaloid tetrandrine with a simple HPLC method in rabbits. Fitoterapia. 2011-09, 82 (6): 878–882. doi:10.1016/j.fitote.2011.04.014.
- ^ 楊敬,朱安祥,胡軍,等. 粉防己鹼的作用機制研究進展[J]. 國際中醫中藥雜誌,2018,40:(3):286-289. DOI:10.3760/cma.j.issn.1673-4246.2018.03.025
- ^ Zhang, Lijin; Geng, Yanling; Duan, Wenjuan. Ionic liquid-based ultrasound-assisted extraction of fangchinoline and tetrandrine from Stephaniae tetrandrae. Journal of Separation Science. 2009-10, 32 (20): 3550–3554 [2021-11-10]. ISSN 1615-9314. PMID 19764054. doi:10.1002/jssc.200900413. (原始內容存檔於2022-06-17).
- ^ 謝林艷,陸嬌,柏芳芳,等. 粉防己鹼:一種治療支氣管哮喘的雙苄基異喹啉類生物鹼[J]. 國際呼吸雜誌,2015,35:(03):210-214. DOI:10.3760/cma.j.issn.1673-436X.2015.03.013
- ^ 6.0 6.1 Kwan, Chiu-Yin; Achike, F. I. Tetrandrine and related bis-benzylisoquinoline alkaloids from medicinal herbs: cardiovascular effects and mechanisms of action. Acta Pharmacologica Sinica. 2002-12, 23 (12): 1057–1068 [2021-11-10]. ISSN 1671-4083. PMID 12466042. (原始內容存檔於2022-07-04).
- ^ Feng, Dechun; Mei, Yunhua; Wang, Ying; Zhang, Bianhong; Wang, Chen; Xu, Lingyun. Tetrandrine protects mice from concanavalin A-induced hepatitis through inhibiting NF-kappaB activation. Immunology Letters. 2008-12-22, 121 (2): 127–133 [2021-11-10]. ISSN 1879-0542. PMID 18992279. doi:10.1016/j.imlet.2008.10.001. (原始內容存檔於2021-11-10).
- ^ Liu, Chaoyang; Gong, Ke; Mao, Xin; Li, Wenhua. Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma. International Journal of Cancer. 2011-09-15, 129 (6): 1519–1531 [2021-11-10]. ISSN 1097-0215. PMID 21128229. doi:10.1002/ijc.25817. (原始內容存檔於2021-11-10).
- ^ Cheng, Zhixiang; Wang, Keming; Wei, Jia; Lu, Xiang; Liu, Baorui. Proteomic analysis of anti-tumor effects by tetrandrine treatment in HepG2 cells. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2010-11, 17 (13): 1000–1005 [2021-11-10]. ISSN 1618-095X. PMID 20554191. doi:10.1016/j.phymed.2010.03.018. (原始內容存檔於2021-11-10).
- ^ Deng, Wen-Ying; Luo, Su-Xia; Zhou, Meng-Qiang; Li, Ning; Chen, Xiao-Bing; Han, Li-Li. [The study of anti-tumor effect of Tetrandrine combined with Nedaplatin on human liver cancer cell line 7402]. Zhong Yao Cai = Zhongyaocai = Journal of Chinese Medicinal Materials. 2008-10, 31 (10): 1522–1525 [2021-11-10]. ISSN 1001-4454. PMID 19230406. (原始內容存檔於2021-11-10).
- ^ Kitano, Ai; Yamanaka, Osamu; Ikeda, Kazuo; Ishida-Nishikawa, Iku; Okada, Yuka; Shirai, Kumi; Saika, Shizuya. Tetrandrine suppresses activation of human subconjunctival fibroblasts in vitro. Current Eye Research. 2008-07, 33 (7): 559–565 [2021-11-10]. ISSN 1460-2202. PMID 18600488. doi:10.1080/02713680802220817. (原始內容存檔於2022-06-15).
- ^ Sakurai, Yasuteru; Kolokoltsov, Andrey A.; Chen, Cheng-Chang; Tidwell, Michael W.; Bauta, William E.; Klugbauer, Norbert; Grimm, Christian; Wahl-Schott, Christian; Biel, Martin. Two pore channels control Ebolavirus host cell entry and are drug targets for disease treatment. Science (New York, N.Y.). 2015-02-27, 347 (6225): 995–998 [2021-11-10]. ISSN 0036-8075. PMC 4550587
 . PMID 25722412. doi:10.1126/science.1258758. (原始內容存檔於2022-06-21).
. PMID 25722412. doi:10.1126/science.1258758. (原始內容存檔於2022-06-21).
- ^ Bhakuni, Dewan S.; Jain, Sudha; Singh, Awadhesh N. Biosynthesis of the bisbenzylisoquinoline alkaloid, tetrandrine. Phytochemistry. 1980-01-01, 19 (11): 2347–2350. ISSN 0031-9422. doi:10.1016/S0031-9422(00)91024-0 (英語).
- ^ Tetrandrine | ≥99%(HPLC) | TargetMol | Calcium Channel inhibitor. targetmol.com. [2021-11-10]. 原始內容存檔於2024-09-10.
