碳金屬化反應
外觀
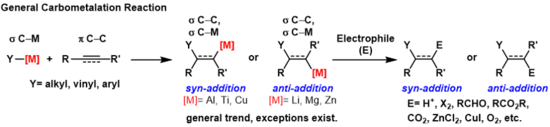
碳金屬化反應(英語:carbometalation)是任何過程中發生以下事件的反應:一個碳-金屬鍵與一個碳-碳π鍵反應,並產生一個新的碳-碳σ鍵與一個碳-金屬σ鍵[1]。 生成的碳金屬鍵可以進行進一步的碳金屬化反應(低聚或聚合,請參見齊格勒-納塔催化劑聚合),也可以使其與各種親電體試劑反應,包括鹵化試劑、羰基化合物、氧和無機鹽,從而生成不同的有機金屬試劑。碳金屬化反應可以在炔烴和烯烴上被進行,以分別形成具有高幾何純度或對映選擇性的產物。
碳鋁化
[編輯]
碳鋁化反應最通常被二氯二茂鋯(或相關催化劑)催化。某些碳鋁化是被用二氯二茂鈦複合物進行的[1] 。該反應有時稱為烯烴的Zr催化不對稱碳鋁化(ZACA)或炔烴的Zr催化甲基鋁化(ZMA)[2] 。
用於該轉化的最常見的三烷基鋁試劑是三甲基鋁,三乙基鋁,有時還有三異丁基鋁。當使用具有β-氫化物的三烷基鋁試劑時,消除反應和氫鋁反應成為競爭過程。
碳鋰化
[編輯]
碳鋰化是在碳-碳pi鍵上添加有機鋰試劑。用於該轉化的有機鋰試劑可以是商用的(例如正丁基鋰),也可以通過去質子化或鹵化鋰交換生成[3][4]。碳鋰化的分子間的和分子內的例子都存在,可被用於合成以產生復雜性。
碳鎂化和碳鋅化
[編輯]由於格氏試劑(有機鎂試劑)和有機鋅試劑的降低的親核性,因此通常僅在活化或應變的烯烴和炔烴上觀察到非催化的碳鎂化和碳鋅化反應[5]。
碳鈀化
[編輯]碳鈀化可以是一個描述由鈀催化劑催化的反應的基本步驟(溝呂木-赫克反應)[6],並且也可以指與鈀催化劑的碳金屬化反應(烯烴雙官能化[7] ,加氫官能化[8][9]) 或還原性[10]))
參考文獻
[編輯]- ^ 1.0 1.1 Negishi, Ei-ichi; Tan, Ze, Diastereoselective, Enantioselective, and Regioselective Carboalumination Reactions Catalyzed by Zirconocene Derivatives, Metallocenes in Regio- and Stereoselective Synthesis: -/-, Topics in Organometallic Chemistry (Springer Berlin Heidelberg), 2005: 139–176, ISBN 9783540314523, doi:10.1007/b96003
- ^ Xu, Shiqing; Negishi, Ei-ichi. Zirconium-Catalyzed Asymmetric Carboalumination of Unactivated Terminal Alkenes. Accounts of Chemical Research. 2016-10-18, 49 (10): 2158–2168. ISSN 0001-4842. PMID 27685327. doi:10.1021/acs.accounts.6b00338.
- ^ O』Shea, Donal F.; Hogan, Anne-Marie L. Synthetic applications of carbolithiation transformations. Chemical Communications. 2008-08-18, (33): 3839–3851 [2020-06-18]. ISSN 1364-548X. PMID 18726011. doi:10.1039/B805595E. (原始內容存檔於2020-07-27).
- ^ García, Graciela V.; Nudelman, Norma Sbarbati. Tandem Reactions Involving Organolithium Reagents. A Review. Organic Preparations and Procedures International. 2009-02-11, 35 (5): 445–500. doi:10.1080/00304940309355860.
- ^ Yorimitsu, Hideki; Murakami, Kei. Recent advances in transition-metal-catalyzed intermolecular carbomagnesiation and carbozincation. Beilstein Journal of Organic Chemistry. 2013-02-11, 9 (1): 278–302. ISSN 1860-5397. PMC 3596116
 . PMID 23503106. doi:10.3762/bjoc.9.34.
. PMID 23503106. doi:10.3762/bjoc.9.34.
- ^ Negishi, Ei-ichi; Copéret, Christophe; Ma, Shengming; Liou, Show-Yee; Liu, Fang. Cyclic Carbopalladation. A Versatile Synthetic Methodology for the Construction of Cyclic Organic Compounds. Chemical Reviews. January 1996, 96 (1): 365–394. ISSN 0009-2665. PMID 11848757. doi:10.1021/cr950020x.
- ^ Sigman, Matthew S.; Jensen, Katrina H. Mechanistic approaches to palladium-catalyzed alkene difunctionalization reactions. Organic & Biomolecular Chemistry. 2008-10-30, 6 (22): 4083–4088. ISSN 1477-0539. PMC 2656348
 . PMID 18972034. doi:10.1039/B813246A.
. PMID 18972034. doi:10.1039/B813246A.
- ^ Engle, Keary M.; McAlpine, Indrawan; Marsters, Rohan P.; Wang, Fen; He, Mingying; Yang, Shouliang; Gallego, Gary M.; Yang, Kin S.; Hill, David E. Palladium(II)-catalyzed γ-selective hydroarylation of alkenyl carbonyl compounds with arylboronic acids. Chemical Science. 2018-11-14, 9 (44): 8363–8368. ISSN 2041-6539. PMC 6247822
 . PMID 30542583. doi:10.1039/C8SC03081B.
. PMID 30542583. doi:10.1039/C8SC03081B.
- ^ O』Duill, Miriam L.; Matsuura, Rei; Wang, Yanyan; Turnbull, Joshua L.; Gurak, John A.; Gao, De-Wei; Lu, Gang; Liu, Peng; Engle, Keary M. Tridentate Directing Groups Stabilize 6-Membered Palladacycles in Catalytic Alkene Hydrofunctionalization. Journal of the American Chemical Society. 2017-11-08, 139 (44): 15576–15579. ISSN 0002-7863. PMC 6002750
 . PMID 28972751. doi:10.1021/jacs.7b08383.
. PMID 28972751. doi:10.1021/jacs.7b08383.
- ^ Gurak, John A.; Engle, Keary M. Practical Intermolecular Hydroarylation of Diverse Alkenes via Reductive Heck Coupling. ACS Catalysis. 2018-10-05, 8 (10): 8987–8992. PMC 6207086
 . PMID 30393575. doi:10.1021/acscatal.8b02717.
. PMID 30393575. doi:10.1021/acscatal.8b02717.
