ELB-139
外观
 | |
| 识别信息 | |
|---|---|
| |
| CAS号 | 188116-08-7 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| 化学信息 | |
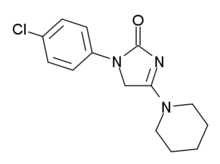
| 化学式 | C14H16ClN3O |
| 摩尔质量 | 277.75 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
ELB-139或LS-191,811是一种含氮有机氯化合物,化学式C
14H
16ClN
3O,能作为非苯二氮䓬类抗焦虑药[1][2]。
它可由1-(4-氯苯基)-2,4-咪唑烷二酮和哌啶在吡啶盐酸盐存在下反应制得。[3]
参考文献
[编辑]- ^ Langen B, Egerland U, Bernöster K, Dost R, Unverferth K, Rundfeldt C. Characterization in rats of the anxiolytic potential of ELB139 [1-(4-chlorophenyl)-4-piperidin-1-yl-1,5-dihydro-imidazol-2-on], a new agonist at the benzodiazepine binding site of the GABAA receptor. The Journal of Pharmacology and Experimental Therapeutics. August 2005, 314 (2): 717–24. PMID 15860576. S2CID 21967108. doi:10.1124/jpet.105.084681.
- ^ Atack JR. The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics. Expert Opinion on Investigational Drugs. May 2005, 14 (5): 601–18. PMID 15926867. S2CID 22793644. doi:10.1517/13543784.14.5.601.
- ^ Grunwald, Christian; et al. Synthesis, Pharmacology, and Structure-Activity Relationships of Novel Imidazolones and Pyrrolones as Modulators of GABAA Receptors. Journal of Medicinal Chemistry (2006), 49(6), 1855-1866. doi:10.1021/jm0509400.
