凝血因子Ⅸ
凝血因子Ⅸ(INN:factor IX,酶学委员会编号3.4.21.22),也称为克里斯马斯因子(英语:Christmas factor),是一种帮助血液凝固的丝氨酸蛋白酶,属于肽酶家族S1。如果缺乏这种蛋白质就会导致B型血友病(也称为克里斯马斯病[6])。
凝血因子Ⅸ是一种血液凝固串级反应中的酶原。人体若是遗传性缺乏或不足会导致出血性疾病 - B型血友病,治疗这种疾病需要持续进行蛋白质替代疗法。[7]
市面上流通的凝血因子Ⅸ是一种纯化的蛋白质,透过基因重组DNA(主要为人类DNA)技术生产。[8]它是一种复杂、经后翻译修饰的人类血清糖蛋白,也是一种高价值的生物制药产品。[9]
此药物一般以药粉形式存在,需要加入特定的溶剂进行稀释后,再透过静脉注射方式给药。[10]
此因子于1952年被发现,当时一个名为史蒂芬·克里斯马斯 (Stephen Christmas) 的美国小男孩被发现缺乏这种因子,而出现血友病。[11]此药物已列入世界卫生组织基本药物标准清单之中。[12]
生理
[编辑]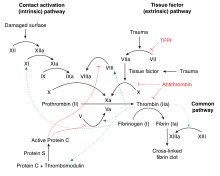
凝血因子Ⅸ是一种酶原,一种无活性的前体。[7]它经过加工去除信号肽,糖基化,然后被第十一a因子(接触调节途径)或第七a因子(组织因子调节途径)裂解,产生双链形式,而双链经由二硫化物连接。[13][14]当激活为第九a因子(factor IXa)时,在 Ca2+、膜磷脂及第八辅因子(VIII cofactor)存在下,它会水解第十因子(factor X)中的一个精氨酸-异亮氨酸键,形成第十a因子(factor Xa)。
抗凝血酶对于凝血因子Ⅸ具有抑制作用,可防止血液过度凝固,形成血栓。[13]
在人类和小鼠中,凝血因子Ⅸ的表达会随着年龄的增长而增加。在小鼠模型中,凝血因子Ⅸ基因启动子区域的突变所引起的表型,会随着年龄的增长而发生变化。[15]
领域架构
[编辑]第七、第九和第十凝血因子均为丝氨酸蛋白酶家族的成员,其共同的结构域使其在催化凝血过程中发挥非常重要的作用。[16]

遗传学
[编辑]
由于第九因子的基因位于X染色体 (Xq27.1-q27.2) 位置上,因此其功能丧失的突变是X连锁隐性遗传:男性比女性更常表现出这种疾病表型。在该基因中已发现至少534种致病突变。[17]凝血因子Ⅸ于1982年由Kotoku Kurachi和美国生化学家艾尔·大卫首次克隆成功。[18]
波莉和茉莉是两只携凝血因子Ⅸ基因的转基因克隆的多赛特绵羊,由英国胚胎学家伊恩·威尔穆特博士于1997年在罗斯林研究所培育成功。[19]
治疗作用
[编辑]| 临床数据 | |
|---|---|
| 商品名 | Benefix |
| 核准状况 | |
| ATC码 |
|
| 法律规范状态 | |
| 法律规范 | |
| 临床数据 | |
|---|---|
| 商品名 | Rixubis |
| 给药途径 | 静脉注射 |
| ATC码 |
|
| 法律规范状态 | |
| 法律规范 |
|
| 临床数据 | |
|---|---|
| 商品名 | Idelvion |
| 核准状况 | |
| ATC码 |
|
| 法律规范状态 | |
| 法律规范 | |
| 临床数据 | |
|---|---|
| 商品名 | Alprolix |
| 核准状况 | |
| ATC码 |
|
| 法律规范状态 | |
| 法律规范 | |
| 临床数据 | |
|---|---|
| 商品名 | Refixia |
| ATC码 |
|
| 法律规范状态 | |
| 法律规范 | |
缺乏凝血因子Ⅸ会导致B型血友病。目前已发现超过3,000种凝血因子Ⅸ的变异,其461个残基中有75%受到影响,[24]有些变异不会引起任何症状,但许多会导致严重的出血性疾病。最初的克里斯马斯病突变是透过对克里斯马斯本人的DNA进行测序而确定,揭示出一个将半胱氨酸变为丝氨酸的突变。[25]
副作用
[编辑]通常给药后的副作用有注射部位疼痛、寒颤、刺痛、潮红、头痛、恶心或呕吐。严重的副作用有胸痛、呼吸困难及指尖发绀。罕见的副作用有严重过敏反应。[26]
特定群体
[编辑]怀孕
[编辑]个体在怀孕期间,非有必要才能使用此药物,应与医师咨询,经过权衡后再做决定。[26]
母乳哺育
[编辑]目前尚无关于哺乳期间使用凝血因子Ⅸ的临床数据。使用前应咨询医师。[27]
禁忌症
[编辑]使用本药物时应谨慎,尤其对于肝病患者、术后患者、肿瘤患者或有血栓或弥漫性血管内凝血风险的患者。应权衡使用的益处与这些并发症的风险。 [8]
市售配方
[编辑]凝血因子Ⅸ经由重组DNA及纯化后而得,用于治疗B型血友病。市售的配方有:
- 非那凝α (nonacog alfa,商品名称Benefix)[28]
- 非那凝γ(nonacog gamma,商品名称Rixubis(立速止))[20]
- albutrepenonacog alfa(商品名称Idelvion(爱必凝))[29]
- eftrenonacog alfa(商品名称Alprolix(爱普力克))[30]
- nonacog beta pegol(商品名称Refixia(瑞菲克))[31]
- 重组凝血因子Ⅸ(商品名称Benefix(宾凝适))[32]
- 重组凝血因子Ⅸ(商品名称Idelvion)[33]
- 重组凝血因子Ⅸ - F融合蛋白(商品名称Alprolix)[34]
- 重组凝血因子Ⅸ(商品名称Ixinity)[35][36]
- 重组凝血因子Ⅸ(商品名称Rebinyn)[37]
- 重组凝血因子Ⅸ(商品名称Rixubis)[38]
- 人类凝血因子Ⅸ(商品名称:Alphanine SD)[39]
凝血因子Ⅸ的一些罕见突变会导致凝血活性升高,而可能导致凝血疾病,例如深静脉血栓。这种功能突变造成蛋白质功能亢进,并与家族性早发血栓形成倾向有关联。[40]
凝血因子Ⅸ缺乏症的治疗方法是注射经由动物或动物细胞载体殖株而产生的纯化凝血因子Ⅸ。对有遗传性第九因子缺乏症,又接受手术的患者,使用传明酸可能有助于降低围手术期间的出血风险。[41]
凝血因子Ⅸ中突变的清单由欧洲血友病与相关疾病协会(European Association for Haemophilia and Allied Disorders,简称EAHAD) 编制和维护。[42]
本药物已列入世界卫生组织基本药物标准清单之中。[12]
参考文献
[编辑]- ^ 與第9凝血因子相關的疾病;在維基數據上查看/編輯參考.
- ^ 2.0 2.1 2.2 GRCh38: Ensembl release 89: ENSG00000101981 - Ensembl, May 2017
- ^ 3.0 3.1 3.2 GRCm38: Ensembl release 89: ENSMUSG00000031138 - Ensembl, May 2017
- ^ Human PubMed Reference:. National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Mouse PubMed Reference:. National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Hemophilia B. SpicyIP. [2024-11-15].
- ^ 7.0 7.1 Orlova, N A; Kovnir, S V. Coagulation Factor IX for Hemophilia B Therapy . Acta Naturae. April 2012, 4 (2): 62–73 [2024-11-16].
- ^ 8.0 8.1 Factor IX. Pediatric Oncall. [2024-11-15].
- ^ Zacchi, Lucia F.; Roche-Recinos, Dinora. Coagulation factor IX analysis in bioreactor cell culture supernatant predicts quality of the purified product. Communications Biology. 2021-03-23, 4 (390(2021)) [2024-11-16].
- ^ Factor IX Concentrates injection. Cleveland Clinic. [2024-11-15].
- ^ Biggs R, Douglas AS, Macfarlane RG, Dacie JV, Pitney WR. Christmas disease: a condition previously mistaken for haemophilia. British Medical Journal. Dec 1952, 2 (4799): 1378–82. PMC 2022306
 . PMID 12997790. doi:10.1136/bmj.2.4799.1378.
. PMID 12997790. doi:10.1136/bmj.2.4799.1378.
- ^ 12.0 12.1 World Health Organization. World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. hdl:10665/325771
 . WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ 13.0 13.1 Di Scipio RG, Kurachi K, Davie EW. Activation of human factor IX (Christmas factor). The Journal of Clinical Investigation. Jun 1978, 61 (6): 1528–38. PMC 372679
 . PMID 659613. doi:10.1172/JCI109073.
. PMID 659613. doi:10.1172/JCI109073.
- ^ Taran LD. Factor IX of the blood coagulation system: a review. Biochemistry. Biokhimiia. Jul 1997, 62 (7): 685–93. PMID 9331959.
- ^ Boland EJ, Liu YC, Walter CA, Herbert DC, Weaker FJ, Odom MW, Jagadeeswaran P. Age-specific regulation of clotting factor IX gene expression in normal and transgenic mice. Blood. Sep 1995, 86 (6): 2198–205. PMID 7662969. doi:10.1182/blood.V86.6.2198.bloodjournal8662198
 .
.
- ^ Zhong D, Bajaj MS, Schmidt AE, Bajaj SP. The N-terminal epidermal growth factor-like domain in factor IX and factor X represents an important recognition motif for binding to tissue factor. The Journal of Biological Chemistry. Feb 2002, 277 (5): 3622–31. PMID 11723140. doi:10.1074/jbc.M111202200
 .
.
- ^ Šimčíková D, Heneberg P. Refinement of evolutionary medicine predictions based on clinical evidence for the manifestations of Mendelian diseases. Scientific Reports. December 2019, 9 (1): 18577. Bibcode:2019NatSR...918577S. PMC 6901466
 . PMID 31819097. doi:10.1038/s41598-019-54976-4.
. PMID 31819097. doi:10.1038/s41598-019-54976-4.
- ^ Kurachi K, Davie EW. Isolation and characterization of a cDNA coding for human factor IX. Proceedings of the National Academy of Sciences of the United States of America. Nov 1982, 79 (21): 6461–4. Bibcode:1982PNAS...79.6461K. PMC 347146
 . PMID 6959130. doi:10.1073/pnas.79.21.6461
. PMID 6959130. doi:10.1073/pnas.79.21.6461  .
.
- ^ Nicholl D. An Introduction to Genetic Engineering Second Edition. Cambridge University Press. 2002: 257.
- ^ 20.0 20.1 Rixubis EPAR. European Medicines Agency (EMA). 19 December 2014 [1 June 2024].
- ^ Health Canada New Drug Authorizations: 2016 Highlights. Health Canada. 2017-03-14 [7 April 2024].
- ^ Alprolix EPAR. European Medicines Agency (EMA). 2007-06-08 [7 June 2024].
- ^ Refixia (Novo Nordisk Pharmaceuticals Pty Ltd). Therapeutic Goods Administration (TGA). 13 September 2024 [2024-09-15].
- ^ Goodeve, A. C. Hemophilia B: Molecular pathogenesis and mutation analysis. Journal of Thrombosis and Haemostasis. 2015, 13 (7): 1184–1195. PMC 4496316
 . PMID 25851415. doi:10.1111/jth.12958.
. PMID 25851415. doi:10.1111/jth.12958.
- ^ Taylor SA, Duffin J, Cameron C, Teitel J, Garvey B, Lillicrap DP. Characterization of the original Christmas disease mutation (cysteine 206----serine): from clinical recognition to molecular pathogenesis. Thrombosis and Haemostasis. Jan 1992, 67 (1): 63–5. PMID 1615485. S2CID 25251813. doi:10.1055/s-0038-1648381.
- ^ 26.0 26.1 How to use Coagulation Factor IX Solution, Reconstituted (Recon Soln). WebMD. [2024-11-15].
- ^ Coagulation Factor IX. Drugs and Lactation Database (LactMed®) [Internet]. 2018-12-03 [2024-11-15].
- ^ Benefix EPAR. European Medicines Agency (EMA). 2018-09-17 [2020-06-17]. (原始内容存档于2020-06-17).
- ^ Idelvion EPAR. European Medicines Agency (EMA). 2018-09-17 [2020-06-17]. (原始内容存档于2020-06-17).
- ^ Alprolix EPAR. European Medicines Agency (EMA). 2018-09-17 [2020-06-17]. (原始内容存档于2020-08-11).
- ^ Refixia EPAR. European Medicines Agency (EMA). 2018-09-17 [2020-06-17]. (原始内容存档于2020-06-18).
- ^ Benefix (coagulation factor ix- recombinant kit. DailyMed. 2023-03-01 [2024 -03-23]. (原始内容存档于2023-01-29).
- ^ Idelvion- coagulation factor ix recombinant human kit. DailyMed. 2023-06-30 [2024-03-23]. (原始内容存档于2023-01-27).
- ^ Alprolix (coagulation factor ix- recombinant, fc fusion protein kit. DailyMed. 2023-05-25 [2024-03-23]. (原始内容存档于2023-02-07).
- ^ Ixinity (coagulation factor ix- recombinant kit. DailyMed. 2021-02-23 [2024-03-23]. (原始内容存档于2023-09-28).
- ^ Ixinity (coagulation factor ix- recombinant kit. DailyMed. 2024-01-09 [2024-03-23]. (原始内容存档于2022-12-03).
- ^ Rebinyn ((coagulation factor ix- recombinant, glycopegylated kit. DailyMed. 2022-08-11 [2024-03-23]. (原始内容存档于2022-11-29).
- ^ Rixubis (coagulation factor ix- recombinant kit. DailyMed. 2023-03-22 [2024-03-23]. (原始内容存档于2022-07-02).
- ^ Alphanine SD (coagulation factor ix- human kit. DailyMed. 2024-01-18 [2024-03-23]. (原始内容存档于2024-02-18).
- ^ Simioni P, Tormene D, Tognin G, Gavasso S, Bulato C, Iacobelli NP, Finn JD, Spiezia L, Radu C, Arruda VR. X-linked thrombophilia with a mutant factor IX (factor IX Padua). The New England Journal of Medicine. Oct 2009, 361 (17): 1671–5. PMID 19846852. doi:10.1056/NEJMoa0904377
 . hdl:11577/2438365
. hdl:11577/2438365  .
.
- ^ Rossi M, Jayaram R, Sayeed R. Do patients with haemophilia undergoing cardiac surgery have good surgical outcomes?. Interactive Cardiovascular and Thoracic Surgery. Sep 2011, 13 (3): 320–31. PMID 21712351. doi:10.1510/icvts.2011.272401
 .
.
- ^ Home: EAHAD Factor 9 Gene Variant Database. [2020-10-23]. (原始内容存档于2020-10-28).
延伸阅读
[编辑]- Davie EW, Fujikawa K. Basic mechanisms in blood coagulation. Annual Review of Biochemistry. 1975, 44: 799–829. PMID 237463. doi:10.1146/annurev.bi.44.070175.004055.
- Sommer SS. Assessing the underlying pattern of human germline mutations: lessons from the factor IX gene. FASEB Journal. Jul 1992, 6 (10): 2767–74. PMID 1634040. S2CID 15211597. doi:10.1096/fasebj.6.10.1634040
 .
. - Lenting PJ, van Mourik JA, Mertens K. The life cycle of coagulation factor VIII in view of its structure and function. Blood. Dec 1998, 92 (11): 3983–96. PMID 9834200. doi:10.1182/blood.V92.11.3983.
- Lowe GD. Factor IX and thrombosis (PDF). British Journal of Haematology. Dec 2001, 115 (3): 507–13 [2019-12-11]. PMID 11736930. S2CID 44650866. doi:10.1046/j.1365-2141.2001.03186.x
 . (原始内容存档 (PDF)于2021-06-19).
. (原始内容存档 (PDF)于2021-06-19). - O'Connell NM. Factor XI deficiency--from molecular genetics to clinical management. Blood Coagulation & Fibrinolysis. Jun 2003, 14 (Suppl 1): S59–64. PMID 14567539. doi:10.1097/00001721-200306001-00014.
- Du X. Signaling and regulation of the platelet glycoprotein Ib-IX-V complex. Current Opinion in Hematology. May 2007, 14 (3): 262–9. PMID 17414217. S2CID 39904506. doi:10.1097/MOH.0b013e3280dce51a.