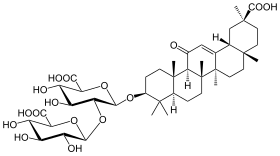
甘草酸
 | |
 | |
| 临床资料 | |
|---|---|
| 商品名 | Epigen, Glycyron |
| AHFS/Drugs.com | 国际药品名称 |
| 给药途径 | 口服、静脉注射 |
| ATC码 | |
| 药物动力学数据 | |
| 药物代谢 | 肝脏和肠道细菌 |
| 生物半衰期 | 6.2-10.2 h[1] |
| 排泄途径 | 粪便、尿液 (0.31-0.67%)[2] |
| 识别信息 | |
| |
| CAS号 | 1405-86-3(α-D-Glucopyranosiduronic acid) 103000-77-7(β-D-Glucopyranosiduronic acid) |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| E编码 | E958 (glazing agents, ...) |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.014.350 |
| 化学信息 | |
| 化学式 | C42H62O16 |
| 摩尔质量 | 822.93 g/mol |
| 3D模型(JSmol) | |
| 水溶性 | 1-10 mg/mL (20 °C) |
| |
| |
甘草酸(Glycyrrhizic acid 或 glycyrrhizinic acid)又称甘草皂苷、甘草甜素(Glycyrrhizin),是甘草(Glycyrrhiza glabra)根的主要甜味成分;在结构上是一种皂苷。可用作食品和化妆品中的乳化剂和凝胶形成剂。其糖苷配基是甘草次酸,在日本被用于降低慢性丙型肝炎患者患肝癌的风险的前体药物。[3] [4]
不良影响
[编辑]通过食用黑甘草使用甘草酸的最广泛报道的副作用是降低血钾水平,对体液平衡和神经功能有影响。[5] [6] 长期服用黑甘草,即使是适量,也会导致血压升高, [6]可能导致心律不齐 ,以及与处方药的不良反应。 [5]
其对体液的影响与肾脏内皮质醇代谢的抑制,和随后对盐皮质激素受体的刺激, [7]以及血液中肾素、钾和醛固酮的水平降低有关,这些因素共同导致血压升高。 [6]
根据摄取黑甘草的量和频率,其他副作用可能包括: [5] [8]
研究
[编辑]正在对甘草酸进行实验室和初步临床研究,以确定其对常见病毒 (如丙型肝炎)的可能活性。[4] 甘草甜素在体外抑制酶11β-羟基类固醇脱氢酶 。 [8]
药代动力学
[编辑]口服摄入后,甘草酸首先被肠道细菌水解为18β-甘草次酸(Enoxolone)。 从肠道完全吸收后,18β-甘草次酸被代谢成肝脏中的3β-单葡糖醛酸-18β-甘草次酸。然后该代谢物在血流中循环。主要部分被胆汁清除,只有一小部分(0.31-0.67%)被尿液清除。 [9] 口服摄入600mg甘草酸后,1.5至14小时后尿液中出现代谢物。1.5至39小时后达到最大浓度(0.49至2.69mg / l),并且在2至4天后仍可在尿液中检测到代谢物。[9]
调味特性
[编辑]甘草酸是甘草根浸提后,在水中煮沸而得的提取物。[10] 甘草提取物(甘草酸)在美国作为液体、糊剂或喷雾干燥粉末出售。 [10] 当在规定量范围时,它被批准用作制造食品,饮料,糖果, 膳食补充剂和调味品中的香料和香味剂 。 [10]它的甜度是蔗糖(食糖)的30至50倍。 [8] [11]
请参见
[编辑]参考文献
[编辑]- ^ van Rossum, TG; Vulto, AG; Hop, WC; Schalm, SW. Pharmacokinetics of intravenous glycyrrhizin after single and multiple doses in patients with chronic hepatitis C infection.. Clinical Therapeutics. December 1999, 21 (12): 2080–90. PMID 10645755. doi:10.1016/S0149-2918(00)87239-2. hdl:1765/73160.
- ^ Ploeger, B; Mensinga, T; Sips, A; Seinen, W; Meulenbelt, J; DeJongh, J. The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling.. Drug Metabolism Reviews. May 2001, 33 (2): 125–47. PMID 11495500. doi:10.1081/DMR-100104400.
- ^ Arase, Yasuji; Ikeda, Kenji; Murashima, Naoya; Chayama, Kazuaki; Tsubota, Akihito; Koida, Isao; Suzuki, Yoshiyuki; Saitoh, Satoshi; Kobayashi, Masahiro. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 15 April 1997, 79 (8): 1494–1500. doi:10.1002/(SICI)1097-0142(19970415)79:8<1494::AID-CNCR8>3.0.CO;2-B.
- ^ 4.0 4.1 Fiore, C; Eisenhut, M; Krausse, R; Ragazzi, E; Pellati, D; Armanini, D; Bielenberg, J. Antiviral effects of Glycyrrhiza species. Phytotherapy Research. 2008, 22 (2): 141–8. PMID 17886224. doi:10.1002/ptr.2295.
- ^ 5.0 5.1 5.2 Black Licorice: Trick or Treat?. US Food and Drug Administration. 30 October 2017 [15 December 2017]. (原始内容存档于2017-06-30). 引用错误:带有name属性“fda-cons”的
<ref>标签用不同内容定义了多次 - ^ 6.0 6.1 6.2 Penninkilampi, R; Eslick, E. M; Eslick, G. D. The association between consistent licorice ingestion, hypertension and hypokalaemia: A systematic review and meta-analysis. Journal of Human Hypertension. 2017, 31 (11): 699–707. PMID 28660884. doi:10.1038/jhh.2017.45.
- ^ Ferrari, P.; Sansonnens, A.; Dick, B.; Frey, F. J. In Vivo 11 -HSD-2 Activity: Variability, Salt-Sensitivity, and Effect of Licorice. Hypertension. 2001, 38 (6): 1330–6. PMID 11751713. doi:10.1161/hy1101.096112.
- ^ 8.0 8.1 8.2 Asl, MN; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds.. Phytotherapy Research. 1 June 2008, 22 (6): 709–24. PMID 18446848. doi:10.1002/ptr.2362.
- ^ 9.0 9.1 Kočevar Glavač, Nina; Kreft, Samo. Excretion profile of glycyrrhizin metabolite in human urine. Food Chemistry. 2012, 131: 305–308. doi:10.1016/j.foodchem.2011.08.081.
- ^ 10.0 10.1 10.2 Sec. 184.1408 Licorice and licorice derivatives. US Food and Drug Administration, Code of Federal Regulations Title 21, 21CFR184.1408. 1 April 2017 [15 December 2017]. (原始内容存档于2021-01-22).
- ^ Glycyrrhizic Acid. PubChem. National Institutes of Health. [24 February 2014]. (原始内容存档于2014-03-11).
